
Californian firm Yali Bio has successfully produced a high-purity breast milk fat using precision fermentation, in a potential breakthrough for the baby formula market.
US food tech startup Yali Bio has hit a critical milestone in its journey to modernise the infant formula industry.
It has produced a precision-fermented breast milk fat with purity levels matching those in human milk, a feat that surprised its R&D team itself.
The fat is called OPO (short for Oleic-Palmitic-Oleic), which is chemically known as 1,3-dioleoyl-2-palmitoyl-glycerol and naturally found in breast milk. It’s an essential component for infant health, allowing them to absorb nutrients, and serves as one of the key nutritional differences between breast milk and baby formula.
Part of the two-year project was funded by a $370,000 grant from the National Institutes of Health’s (NIH) Eunice Kennedy Shriver National Institute of Child Health and Human Development. Yali Bio’s breakthrough comes amid the Trump administration’s funding cuts for the NIH and simultaneous efforts to boost the domestic infant formula supply through Operation Stork Speed.
How Yali Bio scaled the OPO problem
Fats account for around 50% of the energy in breast milk and are crucial for infant growth and development. There is already wide recognition of the cognitive, visual, and immune benefits of ARA and DHA, the two primary long-chain omega-6 and omega-3 fatty acids, respectively.
Precision fermentation, which entails inserting a DNA sequence into microbes to teach them to produce specific compounds when fermented, has already enabled industry giants like DSM to scale up ARA and DHA for global adoption in infant formulas.
OPO, the main structural fat in human milk, is mostly produced by specialty fat companies like Bunge, AAK and Wilmar through an enzymatic manufacturing process using vegetable oils. This form of the fat has been commercialised in major Chinese baby formula products, but remains absent in the US and Europe.
According to Yali Bio, the complexity of the production process, the reliance on high-purity palm fractions, and the high cost of enzymes have limited the adoption of the ingredient in Western markets.
It uses a recombinant yeast platform to efficiently produce high-quality structured fats that resemble breast milk fats in a cost-effective process. At scale, this tech can allow manufacturers to put more OPO in their formula products and potentially present better health outcomes for infants.
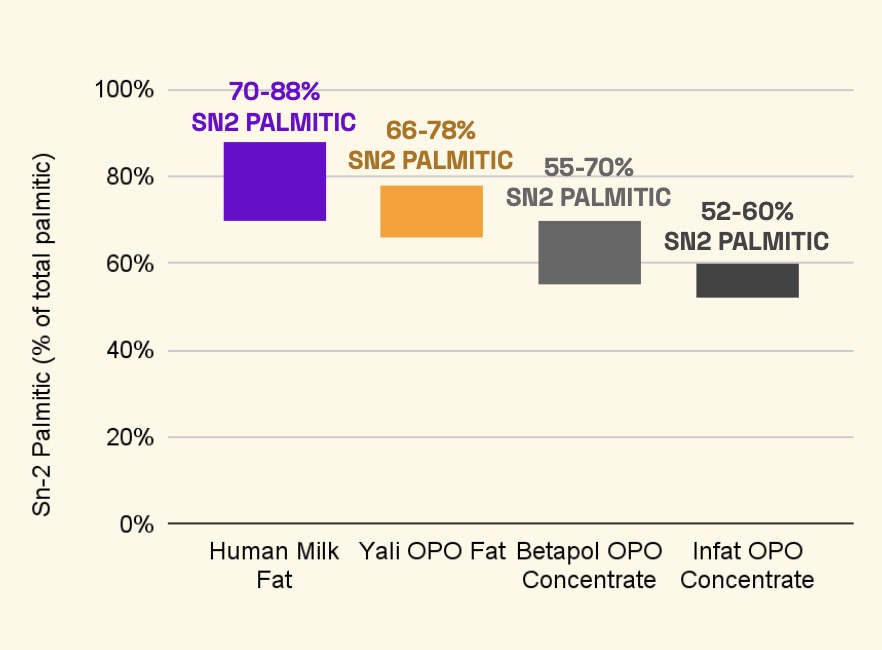
Now, it has produced OPO with a “very high purity level”, as founder and CEO Yulin Lu put it. In breast milk, the levels of palmitic acid can range from 60% to 88% in the middle position of the glycerol backbone (sn-2). Yali Bio’s yeast-derived version reached a 66-78% purity level.
In comparison, two commercially available OPO products using the traditional enzymatic process, Betapol and Infat, have sn-2 palmitic acid levels ranging from 55-70% and 52-60%, respectively.
It could pave the way for widespread adoption of OPO in infant formula, with the ingredient shown to be crucial in fat regulation and calcium absorption, both of which are vital for energy and bone growth during an infant’s first 1,000 days.
Infant formula alternatives under the policy spotlight

“The technology has been advanced to a very reproducible fermentation scale in our laboratory, with an integrated downstream process developed to make the final product, purified OPO,” said Yu.
Yali Bio said it was “learning a great deal about infant nutrition” and “the significant science-based approach and rigour the broader industry has been committed to for novel bioactive ingredients development”. It now has commercial interest from North America, Europe and Asia, and is in “close engagement” with infant formula manufacturers for potential partnerships.
Most of the world’s 130 million babies born annually are fed infant formula at some point. Research has shown that about 5-10% of women are physiologically unable to breastfeed, and many more say they’re not producing enough or have nutritional deficiencies in their milk. According to the Centers for Disease Control and Prevention, only a quarter of American mothers continue to exclusively breastfeed at six months, which is the recommended period by the American Academy of Pediatrics.
It underscores the importance of the consistent supply of nutritionally sound infant formula. Operation Stork Speed aims to boost the quality, safety and nutritional adequacy of these products to help avoid situations like the national shortage in 2022.
The Food and Drug Administration (FDA) has been criticised for its “outdated” regulations around alternative formulas, which have slowed the commercialisation of new products that address allergies and nutritional gaps. Yali Bio has “key milestones planned” for its Generally Recognized as Safe (GRAS) filing with the FDA.
“Amidst changes and stress in federal agency funding at NIH for biomedical research this year, we see […] global food and infant nutrition industry key stakeholders’ strong engagement to advance innovation, to make a greater scientific and public health impact for early nutrition,” said Yu.
Boston-based The Live Green Co is also working on precision-fermented OPO and California’s Checkerspot has developed a microalgae-derived fat analogue to mimic OPO. Portugal’s PFx Biotech is working on human milk proteins made from precision fermentation, including lactoferrin.
US startup Helaina has already launched its recombinant human lactoferrin in seven consumer products, and Australia’s All G is working to commercialise its own version, having produced it on a grams per litre scale. France’s Nūmi and US-based 108Labs, meanwhile, are developing cell-cultivated breast milk.
Yali Bio has previously unveiled a precision-fermented fat for dairy alternatives, too, as well as a yeast-derived replacement for cocoa butter.
The post Yali Bio’s Yeast-Derived Fat Matches Breast Milk Purity Ahead of FDA GRAS Filing appeared first on Green Queen.
This post was originally published on Green Queen.